Abstract
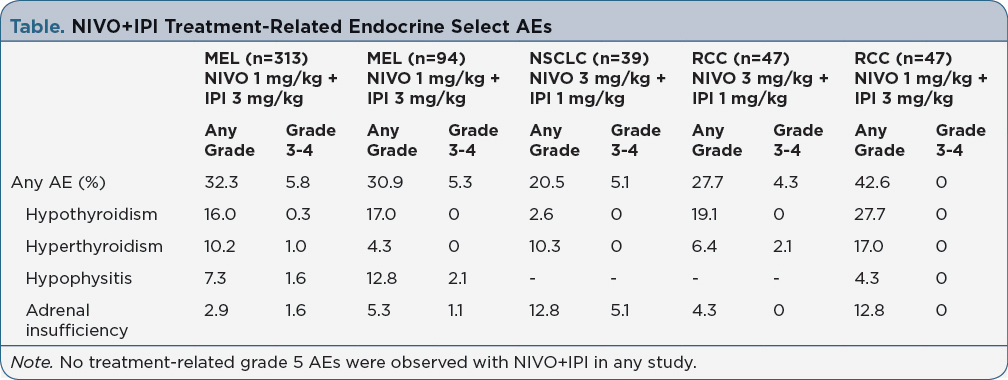
Background: Combination therapy with nivolumab (NIVO) and ipilimumab (IPI) is approved for the first-line treatment of advanced melanoma (MEL). Recent phase 1 studies have also shown promising efficacy with NIVO+IPI in patients (pts) with non-small cell lung cancer (NSCLC) and metastatic renal cell carcinoma (RCC). With NIVO+IPI, select adverse events (ie, immune-related AEs) most commonly affect the skin, gastrointestinal tract, endocrine organs, and liver. Unlike other immune-related AEs, endocrinopathies can persist despite discontinuation or completion of therapy. Here, we review endocrine select AEs associated with NIVO+IPI in solid tumors and provide practical guidance regarding their management. Methods: Safety data were included from phase 2 (CheckMate 069) and phase 3 (CheckMate 067) studies for MEL, and phase 1 studies for NSCLC (CheckMate 012) and RCC (CheckMate 016). Data are reported for NIVO 1 mg/kg + IPI 3 mg/kg Q3W x 4 for MEL, NIVO 3 mg/kg Q2W + IPI 1 mg/kg Q6W for NSCLC, and NIVO 3 + IPI 1 or NIVO 1 + IPI 3 mg/kg Q3W x 4 for RCC, followed by NIVO Q2W. Pts were treated until disease progression, unacceptable toxicity, or withdrawal of consent. Results: In pts with MEL who received NIVO+IPI combination therapy, ~32% experienced any endocrine select AE, of which 5-6% were grade 3-4 (Table). Endocrine select AEs of any grade occurred in 21% of pts with NSCLC, in 28% of pts with RCC who received NIVO 3 + IPI 1 mg/kg, and in 43% of pts with RCC who received NIVO 1 + IPI 3 mg/kg. The most common treatment-related endocrine select AEs across tumor types were hypothyroidism, hyperthyroidism, and adrenal insufficiency. Median time to onset for endocrine select AEs was 6 weeks in MEL. Only 2-3% of pts discontinued due to treatment-related endocrine select AEs. Some pts (typically those with lower grade AEs) can continue NIVO if the AEs were related to IPI. Persistent or worsening fatigue, headaches, and nausea are common symptoms, and may be associated with hypophysitis. Baseline labs (including thyroid-stimulating hormone) should be performed. MRI with pituitary cut can be used to evaluate potential immune-mediated hypophysitis. Pts with thyroid dysfunction need symptomatic management and thyroid hormone replacement, and those with adrenal insufficiency may require long-term glucocorticoid replacement. Implications: Endocrine select AEs are manageable with established treatment algorithms. Given the unique nature of endocrine AEs, early identification is critical and a multidisciplinary team approach is required to effectively manage these pts.